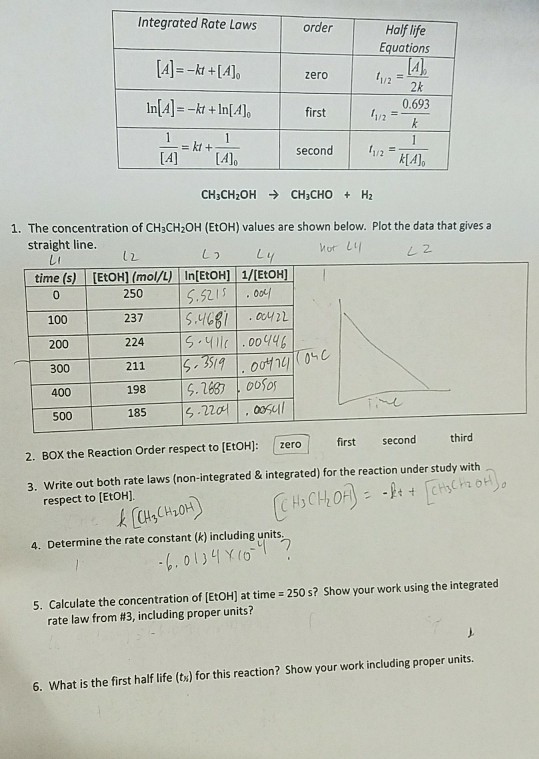
Half Life Equation For Zero . T ½ = 0.693 / k. T ½ = [a o] / 2k. equations for half lives. For a first order reaction a products , rate = k [a]: half life of zero order reactions. For a zero order reaction a products , rate = k: 1st, and 2nd order reactions.
from www.chegg.com
For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: half life of zero order reactions. T ½ = [a o] / 2k. equations for half lives. T ½ = 0.693 / k. 1st, and 2nd order reactions.
Solved Integrated Rate Laws order Half life Equations zero
Half Life Equation For Zero T ½ = [a o] / 2k. T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: equations for half lives. T ½ = [a o] / 2k. 1st, and 2nd order reactions. half life of zero order reactions. For a zero order reaction a products , rate = k:
From www.youtube.com
Understanding Halflife formulas with example. YouTube Half Life Equation For Zero For a first order reaction a products , rate = k [a]: 1st, and 2nd order reactions. For a zero order reaction a products , rate = k: equations for half lives. half life of zero order reactions. T ½ = [a o] / 2k. T ½ = 0.693 / k. Half Life Equation For Zero.
From askfilo.com
Derive Rate equation for zero \& first order Reaction Define half life pe.. Half Life Equation For Zero half life of zero order reactions. equations for half lives. T ½ = 0.693 / k. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. 1st, and 2nd order reactions. For a first order reaction a products , rate = k [a]: Half Life Equation For Zero.
From www.wikihow.com
How to Calculate Half Life 6 Steps (with Pictures) wikiHow Half Life Equation For Zero T ½ = 0.693 / k. half life of zero order reactions. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: 1st, and 2nd order reactions. equations for half lives. Half Life Equation For Zero.
From louratilley.blogspot.com
half life formula for zero order reaction Loura Tilley Half Life Equation For Zero 1st, and 2nd order reactions. half life of zero order reactions. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: equations for half lives. Half Life Equation For Zero.
From dxolokulm.blob.core.windows.net
Half Life Equation Step 1 at Albert Duffy blog Half Life Equation For Zero 1st, and 2nd order reactions. half life of zero order reactions. For a zero order reaction a products , rate = k: equations for half lives. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. T ½ = 0.693 / k. Half Life Equation For Zero.
From www.toppr.com
Derive the relationship between half life period and rate constant of a Half Life Equation For Zero T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: 1st, and 2nd order reactions. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. half life of zero order reactions. equations for half lives. Half Life Equation For Zero.
From byjus.com
Time taken to complete half life of zero order reaction is 75 secs Half Life Equation For Zero half life of zero order reactions. equations for half lives. 1st, and 2nd order reactions. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: Half Life Equation For Zero.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation For Zero 1st, and 2nd order reactions. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. equations for half lives. half life of zero order reactions. Half Life Equation For Zero.
From dxobrspma.blob.core.windows.net
Half Life Equation Variables at Roberto Whitney blog Half Life Equation For Zero half life of zero order reactions. 1st, and 2nd order reactions. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: equations for half lives. T ½ = 0.693 / k. Half Life Equation For Zero.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation For Zero For a zero order reaction a products , rate = k: 1st, and 2nd order reactions. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: equations for half lives. T ½ = 0.693 / k. half life of zero order reactions. Half Life Equation For Zero.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Half Life Equation For Zero For a zero order reaction a products , rate = k: half life of zero order reactions. For a first order reaction a products , rate = k [a]: equations for half lives. 1st, and 2nd order reactions. T ½ = [a o] / 2k. T ½ = 0.693 / k. Half Life Equation For Zero.
From www.youtube.com
Derivation of Half life period of Zero order reaction(chemical Half Life Equation For Zero 1st, and 2nd order reactions. T ½ = 0.693 / k. equations for half lives. half life of zero order reactions. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Half Life Equation For Zero.
From www.chegg.com
Solved Integrated Rate Laws order Half life Equations zero Half Life Equation For Zero T ½ = [a o] / 2k. half life of zero order reactions. equations for half lives. For a zero order reaction a products , rate = k: 1st, and 2nd order reactions. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. Half Life Equation For Zero.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second Half Life Equation For Zero 1st, and 2nd order reactions. For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: T ½ = 0.693 / k. equations for half lives. T ½ = [a o] / 2k. half life of zero order reactions. Half Life Equation For Zero.
From www.tessshebaylo.com
Nuclear Chemistry Half Life Equation Tessshebaylo Half Life Equation For Zero T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: 1st, and 2nd order reactions. equations for half lives. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: half life of zero order reactions. Half Life Equation For Zero.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Half Life Equation For Zero For a zero order reaction a products , rate = k: 1st, and 2nd order reactions. equations for half lives. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. half life of zero order reactions. Half Life Equation For Zero.
From www.youtube.com
Determine the halflife of a zero order reaction YouTube Half Life Equation For Zero half life of zero order reactions. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. equations for half lives. For a first order reaction a products , rate = k [a]: 1st, and 2nd order reactions. T ½ = [a o] / 2k. Half Life Equation For Zero.
From dxolokulm.blob.core.windows.net
Half Life Equation Step 1 at Albert Duffy blog Half Life Equation For Zero equations for half lives. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: half life of zero order reactions. 1st, and 2nd order reactions. T ½ = 0.693 / k. Half Life Equation For Zero.